Diphenhydramine Capsules Prescribing Information
Package insert / product label
Generic name: diphenhydramine hydrochloride
Dosage form: capsule
Drug classes: Anticholinergic antiemetics, Anticholinergic antiparkinson agents, Antihistamines, Miscellaneous anxiolytics, sedatives and hypnotics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
On This Page
Diphenhydramine Capsules Description
Diphenhydramine Hydrochloride is an antihistamine drug having the chemical name 2-(diphenylmethoxy)-N, N-dimethylethylamine hydrochloride. It occurs as a white, odorless crystalline powder and is freely soluble in water and alcohol. The structural formula is as follows:
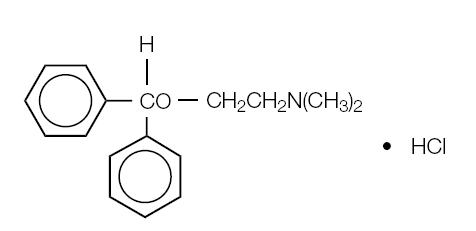
C17H21NO • HCl Molecular Weight: 291.82
Each capsule contains 50 mg of diphenhydramine hydrochloride for oral administration.
Inactive Ingredients:
Anhydrous lactose, lactose monohydrate and magnesium stearate.
The 50 mg capsule shell contains D&C red no. 28, FD&C blue no. 1, FD&C red no. 40, gelatin, silicon dioxide and sodium lauryl sulfate.
The imprinting ink contains D&C yellow no. 10 aluminum lake, FD&C blue no. 1 aluminum lake, FD&C blue no. 2 aluminum lake, FD&C red no. 40 aluminum lake, pharmaceutical glaze, propylene glycol and synthetic black iron oxide.
Diphenhydramine Capsules - Clinical Pharmacology
Diphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative effects. Antihistamines appear to compete with histamine for cell receptor sites on effector cells.
A single oral dose of diphenhydramine hydrochloride is quickly absorbed with maximum activity occurring in approximately one hour. The duration of activity following an average dose of diphenhydramine hydrochloride is from four to six hours. Diphenhydramine is widely distributed throughout the body, including the CNS. Little, if any, is excreted unchanged in the urine; most appears as the degradation products of metabolic transformation in the liver, which are almost completely excreted within 24 hours.
Related/similar drugs
acetaminophen, prednisone, ibuprofen, ondansetron, hydroxyzine, fluticasone nasal, clonazepam
Indications and Usage for Diphenhydramine Capsules
Diphenhydramine hydrochloride in the oral form is effective for the following indications:
Antihistaminic:
For allergic conjunctivitis due to foods; mild, uncomplicated allergic skin manifestations of urticaria and angioedema; amelioration of allergic reactions to blood or plasma; dermatographism; as therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
Antiparkinsonism:
For parkinsonism (including drug-induced) in the elderly unable to tolerate more potent agents; mild cases of parkinsonism (including drug-induced) in other age groups; in other cases of parkinsonism (including drug-induced) in combination with centrally acting anticholinergic agents.
Nighttime sleep-aid.
Contraindications
Use in Nursing Mothers:
Because of the higher risk of antihistamines for infants generally, and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Antihistamines are also contraindicated in the following conditions: Hypersensitivity to diphenhydramine hydrochloride and other antihistamines of similar chemical structure.
Warnings
Antihistamines should be used with considerable caution in patients with narrow-angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy, or bladder-neck obstruction.
Precautions
General:
Diphenhydramine hydrochloride has an atropine-like action and therefore should be used with caution in patients with a history of lower respiratory disease including asthma, increased intraocular pressure, hyperthyroidism, cardiovascular disease or hypertension.
Information for Patients:
Patients taking diphenhydramine hydrochloride should be advised that this drug may cause drowsiness and has an additive effect with alcohol.
Patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.
Drug Interactions:
Diphenhydramine hydrochloride has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc).
MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Long-term studies in animals to determine mutagenic and carcinogenic potential have not been performed.
Pregnancy:
Pregnancy Category B:
Reproduction studies have been performed in rats and rabbits at doses up to 5 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to diphenhydramine hydrochloride. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Adverse Reactions/Side Effects
The most frequent adverse reactions are underscored.
- General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and throat.
- Cardiovascular System: Hypotension, headache, palpitations, tachycardia, extrasystoles.
- Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Nervous System:Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, neuritis, convulsions.
- GI System:Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
- GU System: Urinary frequency, difficult urination, urinary retention, early menses.
- Respiratory System:Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Overdosage
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in children. Atropine-like signs and symptoms, dry mouth; fixed, dilated pupils; flushing; and gastrointestinal symptoms may also occur.
If vomiting has not occurred spontaneously the patient should be induced to vomit. This is best done by having him drink a glass of water or milk after which he should be made to gag. Precautions against aspiration must be taken, especially in infants and children.
If vomiting is unsuccessful gastric lavage is indicated within 3 hours after ingestion and even later if large amounts of milk or cream were given beforehand. Isotonic or (½) isotonic saline is the lavage solution of choice.
Saline cathartics, as milk of magnesia, by osmosis draw water into the bowel and therefore are valuable for their action in rapid dilution of bowel content.
Stimulants should not be used.
Vasopressors may be used to treat hypotension.
Diphenhydramine Capsules Dosage and Administration
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND RESPONSE OF THE PATIENT.
A single oral dose of diphenhydramine hydrochloride is quickly absorbed with maximum activity occurring in approximately one hour. The duration of activity following an average dose of diphenhydramine hydrochloride is from four to six hours.
CHILDREN:
(over 20 lb): 12.5 to 25 mg three to four times daily. Maximum daily dosage not to exceed 300 mg. For physicians who wish to calculate the dose on the basis of body weight or surface area, the recommended dosage is 5 mg/kg/24 hours or 150 mg/m2/24 hours.
Data are not available on the use of diphenhydramine hydrochloride as a nighttime sleep-aid in children under 12 years.
The basis for determining the most effective dosage regimen will be the response of the patient to medication and the condition under treatment.
In motion sickness, full dosage is recommended for prophylactic use, the first dose to be given 30 minutes before exposure to motion and similar doses before meals and upon retiring for the duration of exposure.
How is Diphenhydramine Capsules supplied
Diphenhydramine Hydrochloride Capsules, USP are available as:
| 50 mg: | Pink clear cap/pink clear body filled with white powder. Imprinted in black ink stylized barr 059. | ||||
| Available in bottles of: | |||||
| 100 | NDC 0555-0059-02 | ||||
| 1000 | NDC 0555-0059-05 | ||||
Dispense with a child-resistant closure in a tight container as defined in the USP/NF.
Store at controlled room temperature 15°-30°C (59°-86°F).
| DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsule |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Barr Laboratories Inc. (802716563) |
Frequently asked questions
- What is the max dose of diphenhydramine for adults?
- Does Mucinex help with Covid?
- Does diphenhydramine raise blood pressure?
- Can diphenhydramine be used as a sleep aid?
- Does Benadryl help with or cause anxiety?
- Diphenhydramine Hydrochloride vs Citrate: What's the difference?
More about diphenhydramine
- Check interactions
- Compare alternatives
- Pricing & coupons
- Reviews (531)
- Drug images
- Latest FDA alerts (2)
- Side effects
- Dosage information
- Patient tips
- During pregnancy
- Support group
- Drug class: anticholinergic antiemetics
- Breastfeeding
Patient resources
Professional resources
- DiphenhydrAMINE monograph
- Diphenhydramine (FDA)
- Diphenhydramine Injection (FDA)
- Diphenhydramine Oral Solution (FDA)
Other brands
Benadryl, Benadryl Allergy, Diphen, Dicopanol


